DOSING
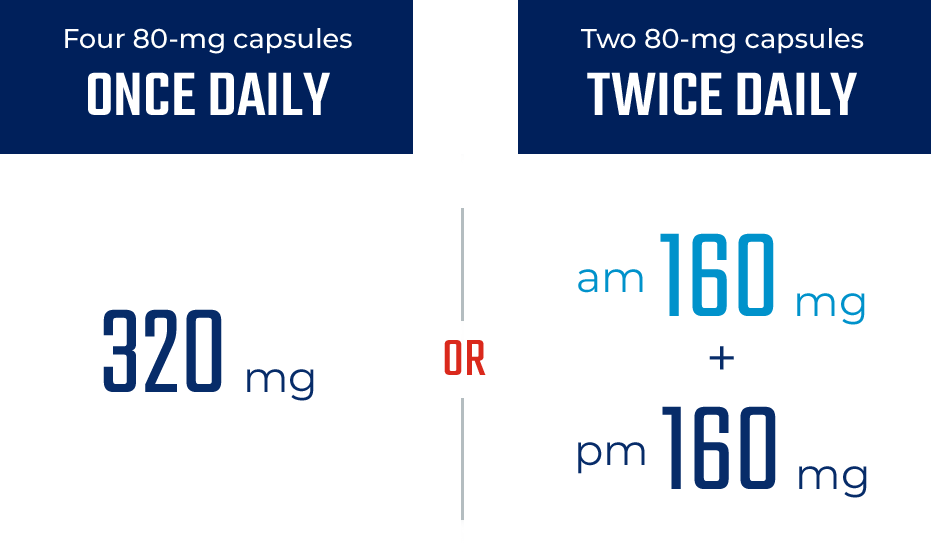
ONCE- OR TWICE-DAILY DOSING1
The only BTKi that can be dosed either ONCE or TWICE daily1
Administration1
- Can be taken with or without food. Can be taken with a high-fat meal – BRUKINSA drug concentration (AUC) is not affected
- Advise patients to swallow capsules whole with water—do not open, dissolve, or chew capsules
- BRUKINSA must not be taken with grapefruit juice, grapefruit, and/or Seville oranges
- If a dose of BRUKINSA is missed, it can be taken as soon as possible with a return to the normal schedule the following day
BRUKINSA should be taken until disease progression or unacceptable toxicity.
- AUC=area under the concentration-time curve.
RECOMMENDED DOSE ADJUSTMENTS1
| CYP | Coadministered drug | Recommended dose |
|---|---|---|
| Inhibition | Strong CYP3A inhibitor (e.g., posaconazole, voriconazole, ketoconazole, itraconazole, clarithromycin, lopinavir, ritonavir, telaprevir) | 80 mg once daily Interrupt dose as recommended for adverse reactions |
| Moderate CYP3A inhibitor (e.g., erythromycin, ciprofloxacin, diltiazem, dronedarone, fluconazole, verapamil, aprepitant) | 80 mg twice daily Modify dose as recommended for adverse reactions |
|
| Induction | Strong CYP3A inducer (e.g., carbamazepine, phenytoin, rifampin) | Avoid concomitant use; consider alternative agents with less CYP3A induction |
| Moderate CYP3A inducer (e.g., bosentan, efavirenz, etravirine, modafinil, nafcillin) | Avoid concomitant use. If concomitant use cannot be avoided, increase BRUKINSA dose to 320 mg twice daily. Monitor closely for toxicity. |
No dose adjustment is recommended in patients with mild to moderate renal or hepatic impairment.
Straightforward Dose Modification for ≥Grade 3 Adverse Reactions (ARs)1
Recommended dose modification for ≥Grade 3 ARs
- Grade ≥3: non-hematological toxicities
- Grade 3: febrile neutropenia/thrombocytopenia with significant bleeding
- Grade 4: neutropenia*/thrombocytopenia*
| Starting Dose | 1st Occurrence | 2nd Occurrence | 3rd Occurrence | 4th Occurrence |
|---|---|---|---|---|
| Start at 320 mg† total dose |
Interrupt BRUKINSA | Interrupt BRUKINSA | Interrupt BRUKINSA | Discontinue |
| Resume BRUKINSA once toxicity has resolved to ≤Grade 1 or baseline |
Resume BRUKINSA once toxicity has resolved to ≤Grade 1 or baseline |
Resume BRUKINSA once toxicity has resolved to ≤Grade 1 or baseline |
||
| No dose reduction. 320 mg† total dose |
Reduce to 160 mg total dose |
Reduce to 80 mg total dose |
- * Lasting >10 consecutive days.
- † 160 mg BID or 320 mg OD.
- ARs=adverse reactions; BID=twice a day; OD=once daily.
Pooled treatment-emergent adverse reactions in ≥10% of patients with hematologic malignancies* (N=1,550)5
| Treatment-Emergent Adverse Reactions | BRUKINSA (N=1,550) | |
|---|---|---|
| All Grades (%) | Grade ≥3 (%) | |
| Upper respiratory tract infection† | 37.1 | 2.2 |
| Bruising† | 30.4 | 0.5 |
| Neutropenia† | 27.5 | 18.5 |
| Haemorrhage/hematoma† | 27.4 | 3.0 |
| Musculoskeletal pain† | 27.0 | 1.8 |
| Rash† | 25.0 | 0.7 |
| Diarrhea | 18.8 | 1.5 |
| Cough† | 18.6 | 0.1 |
| Pneumonia† | 17.9 | 9.4 |
| Fatigue† | 16.2 | 1.2 |
| Thrombocytopenia† | 15.9 | 5.7 |
| Anemia† | 14.1 | 5.2 |
| Hypertension† | 13.0 | 6.5 |
| Constipation | 12.3 | 0.3 |
| Urinary tract infection† | 11.6 | 1.7 |
| Dizziness† | 10.7 | 0.3 |
- * Chronic lymphocytic leukemia, small lymphocytic lymphoma, Waldenström’s macroglobulinemia, mantle cell lymphoma, follicular lymphoma, marginal zone lymphoma, hairy cell leukemia, diffuse large B-cell lymphoma, and Richter’s transformation.
- † Indicates grouped term that includes multiple preferred terms.
Pooled selected treatment-emergent adverse events of special interest in patients with hematologic malignancies* (N=1,550)5
| Treatment-emergent adverse events | All Grades (%) | Grade ≥3 (%) |
|---|---|---|
| Arthralgia | 12.8 | 0.3 |
| Myalgia | 3.9 | 0.3 |
| Atrial fibrillation | 2.9 | 0.8 |
| Atrial flutter | 0.3 | 0.0 |
Of the 1,550 patients treated with BRUKINSA, 3.0% discontinued therapy and 5.2% experienced a dose reduction due to adverse reactions.1
BRUKINSA Ordering Information
BRUKINSA is supplied through Sentrex Health Solutions
For product ordering information, please call 1-833-234-4366
or email info@mybeigene.ca
 Patient Support
Patient Support
Designed to provide appropriate information and assistance to BRUKINSA patients, including:
Reimbursement/payment support
- Dedicated case managers to support insurance verification, bridge, and/or co-pay for patients to gain access to BRUKINSA
Education and support
- Dedicated nurse case managers for practices, patients, and caregivers
- Helps provide information about their disease and treatment with BRUKINSA
- Specialty pharmacists who speak to patients with every refill
Connections to third-party advocacy organizations
- Assists patients and caregivers with practical help through connections to advocacy groups and local/national free resources such as:
- Education and events
- Support group information
THE BEIGENE SAMPLE PROGRAM
BeiGene provides you with samples of BRUKINSA (zanubrutinib) for your patients

Receive a sample in 2 easy steps
- Click “Request Sample” below or request a sample form from your Regional Manager.
- Complete the form and submit.


These are prescription drug samples provided in accordance with Food and Drug Regulations.
It is unlawful to sell, trade, barter, return for credit, utilize to seek reimbursement, or bill patients for samples.

